 Let Breathing Take Control
Let Breathing Take Control
The AEROECLIPSE® II BAN® Nebulizer creates aerosol only in response to the patient’s inspiratory flow.
 Not All Chambers Are Equal
Not All Chambers Are Equal
Some “antistatic” valved holding chambers are only poorly antistatic and are noninterchangeable, which means that switching between them should be discouraged.1
 Improves Patient Outcomes
Improves Patient Outcomes
Recent publications support use of the AEROBIKA® Oscillating Positive Expiratory Pressure device to help reduce readmissions or improve aerosol deposition when used alongside standard of care.2
Recent Poster Presentations

A Risk Evaluation for Fugitive Aerosol Emissions When Using Different Nebulizer Systems: Potential Mechanisms, Impact of Device Type and Mitigation Strategies
This poster provides a review of different nebulizer designs, highlighting the potential for the loss of medicinal and bio-aerosols in their use scenarios. It provides mitigation strategies to greatly reduce or virtually eliminate the potential risks of fugitive emissions associated with nebulized drug delivery.
Failure of Mycobacterium avium to Adhere to Interior Surfaces of Oscillating Positive Expiratory Pressure (OPEP) and Nebulizer Devices
Risk of respiratory infection is a common concern for bronchiectasis patients often leading to caution involving use of medical devices for inhalation. M. avium is a major cause of lung infection in bronchiectasis patients and is common in household drinking water that could be used to rinse respiratory devices.
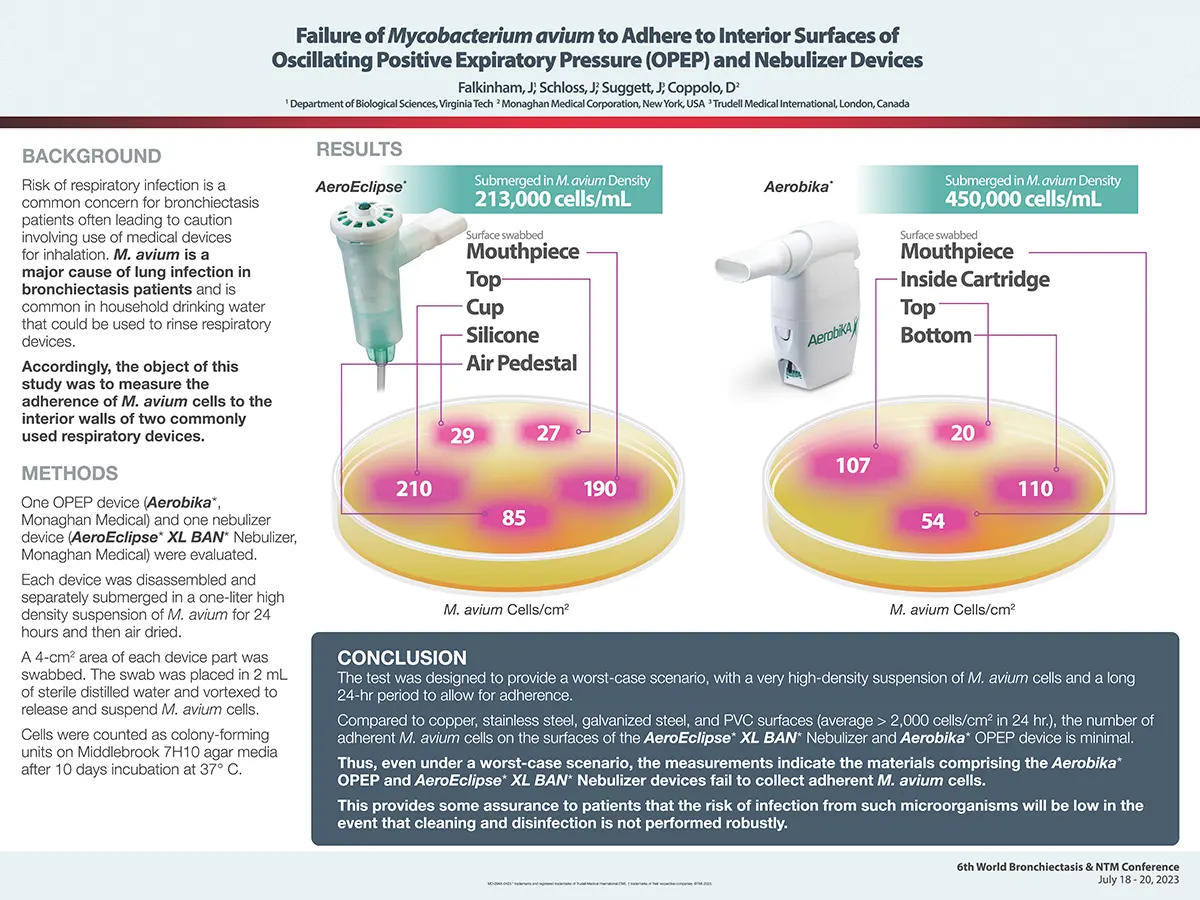

AEROECLIPSE® II BAN® Nebulizer
Let Breathing Take Control
The AEROECLIPSE® II BAN® Nebulizer produces aerosol with a high respirable dose and optimal particle size ONLY when the patient is inhaling. Reducing medication waste and the aerosol particulate that ends up in the room by 3-4 times than that of a continuously run jet nebulizer.3,4 This helps minimize exposure to environmental loss for front line healthcare professionals.
Clinical Evidence
Not all jet nebulizers are created equal. For dose assurance and a safe environment for your frontline staff, choose AEROECLIPSE® II BAN® Nebulizer.
Dose Assurance with Nebulizer Therapy
Continuous output nebulizers risk the potential under-dosing as disease progresses or if a patient pauses during treatment. Clinicians should be aware of the differences between nebulizers and their impact on dose delivery. Watch the video.
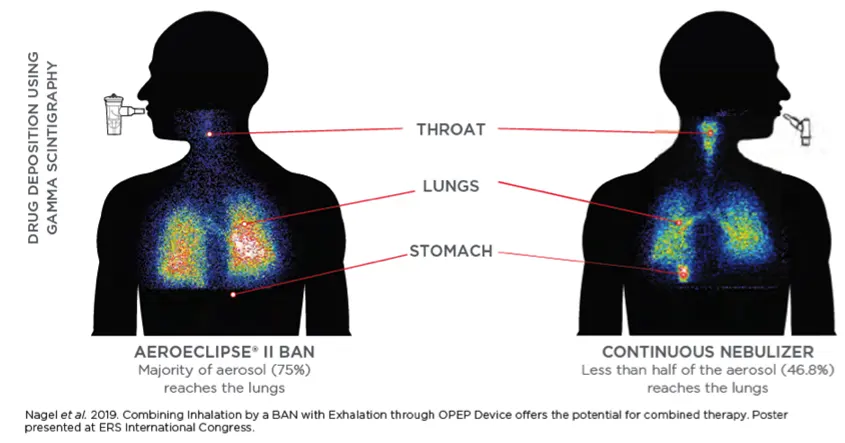
Comparative Scintigraphic Assessment of Deposition of Radiolabeled Albuterol Delivered from a Breath Actuated Nebulizer and a Small Volume Jet Nebulizer to Healthy Subjects
Significantly more aerosol is delivered to the therapeutically important areas of the lungs when compared to a continuously running nebulizer. Learn more.
Implementation of AEROECLIPSE® II BAN® Nebulizer based therapy has the potential to reduce costs associated with staff acquisition of nosocomial influenza
Implementation of the AEROECLIPSE® II BAN® Nebulizer in a hospital Emergency Department resulted in an 88% reduction in the cost of sick days by reducing the number of sick days by 60%. Learn more.
Significant variation exists in the Delivered Dose and the Respirable Dose between different brands of non-breath-activated nebulizer
When different inspiratory:expiratory ratios were considered, there was significant variance in the Delivered Dose and the Respirable Dose between different brands of non-breath-activated nebulizer. Consideration should be given to revision of the test protocols included in the guidelines, to reflect more accurately the potential therapeutic dose that is delivered to a realistic spectrum of breathing patterns. Learn more.
Full Clinical Study Summary
This Study Summary is designed to identify how the AEROECLIPSE® II BAN™ Nebulizer has performed in both in vitro and in vivo studies with various formulations and versus other nebulizers. Learn more.
AEROCHAMBER PLUS® FLOW-VU® AVHC
For respiratory patients, getting the most from life means getting the most from their inhaler medication.
AEROCHAMBER PLUS® brand of chambers delivers the intended dose established by the pharmaceutical company.⁶ Use of other chambers may lead to medication waste, less than optimal doses, and varying therapeutic responses.
Clinical Evidence
AEROCHAMBER PLUS® FLOW-VU® aVHC Clinical Evidence Summary
A quick view of the most recent peer-reviewed evidence supporting the chambers. Learn more
Valved Holding Chambers are not all equivalent in performance and as a result should not be regarded as interchangeable
Only AEROCHAMBER PLUS® FLOW-VU® chambers were equivalent to the reference device data listed in virtually all innovator inhalers currently approved in the US and European markets. Differences in chamber design, materials and function mean that chambers should not be automatically considered interchangeable. Learn more.
Different chambers deliver different amounts of medication which can lead to meaningful differences in clinical performance
The author found that it was unequivocal that differences exist between different chambers which in a number of cases are sufficiently large that meaningful and overt clinical differences would be anticipated as a result. Learn more.
A comparative analysis of changes in pMDI drug dose delivery before and after detergent coating using five antistatic valved holding chambers.
Some “antistatic” valved holding chambers (aVHC) have poor antistatic properties and should therefore be considered non-antistatic and primed. Differences in antistatic properties are an important cause of the large performance differences between aVHCs. Learn more.
For a copy of our full Clinical Study Summary, please contact your Territory Manager.


AEROBIKA® OPEP Device
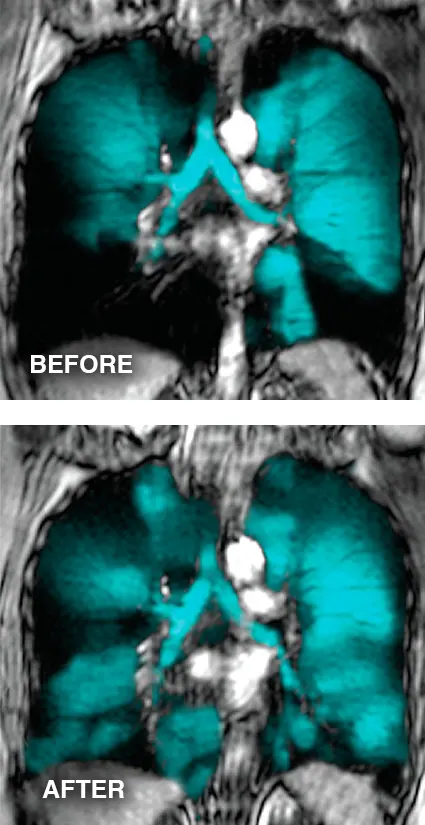
Oscillating Positive Expiratory Pressure or OPEP therapy helps stent open and clear blocked airways. On exhalation the device creates a unique oscillation and pressure dynamic to address the structural and functional challenges in the airways. The combination of oscillations and pressure opens the airways and aids in moving mucus into the central airways, where it can be coughed out. The device is drug-free so there are no side effects or drug interactions and it is easy-to-use, so patients are able to transition home with the device.
Clinical Evidence
Airway Clearance in the COVID-19 Treatment Pathway
An evidence assessment for the integration of the AEROBIKA® OPEP device into the care pathway for COVID-19 patients with airway clearance needs. Watch the video.
AEROBIKA® OPEP Device Clinical Evidence Summary
A quick view of the most recent peer-reviewed evidence supporting the AEROBIKA® OPEP Device. Learn more.
Use of the AEROBIKA® OPEP device resulted in decreased readmissions versus standard of care
- 28% decrease in readmissions for COPD exacerbations. Learn more.
- 39% decrease in all-cause re-hospitalizations and lower costs for post-operative recovery. Learn more.
Contact your Territory Manager today for a copy of our full Clinical Study Summary for the AEROBIKA® OPEP device.
Words or phrases accompanied by ™ and ® are trademarks and registered trademarks of Monaghan Medical Corporation or an affiliate of Monaghan Medical Corporation.
References
1. Hagedoorn P, et al. J Aller Clin Immunol 2020;8(3): 1124-1125e4.
2. Burudpakdee C, et al. Pulmonary Therapy 2017;3(1):163-171; Burudpakdee C, et al. Pulmonary Therapy 2018;4(1):87-101; and Leemans G, et al. COPD 2020;15: 1261–1268.
3. Rau, JL, et al. Performance Comparison of Nebulizer Designs: Constant-Output, Breath-Enhanced, and Dosimetric. Resp Care 2004;(49):174-179 and TMI Aerosol Lab, data on file (600cc, 10BPM, 1:2, Albuterol Sulfate Inhalation Solution 833µg/mL).
4. Nagel M, et al. Am J Respir Crit Care Med 2021;203:A4672.
5. Nagel M, Suggett J, Mitchell JP. RDD 2021;1:287-292.