Nagel M,1 Ali R,1 Doyle C,1 Suggett J,1 Coppolo D2
1 Trudell Medical International, London, Ontario, Canada 2 Monaghan Medical, Syracuse, NY, United States
RATIONALE
Nebulization is the mainstay of care for patients requiring inhaled antibiotic therapy in association with pulmonary diseases such as cystic fibrosis (CF), bronchiectasis and chronic obstructive lung disease (COPD).
Breath actuated (BA) technology offers more consistent dose delivery1 and the reduction of fugitive emissions2 into the care environment. This in vitro study was undertaken to determine delivery of tobramycin using a BA nebulizer/compressor system and two breath enhanced (BE) nebulizer/compressor systems.
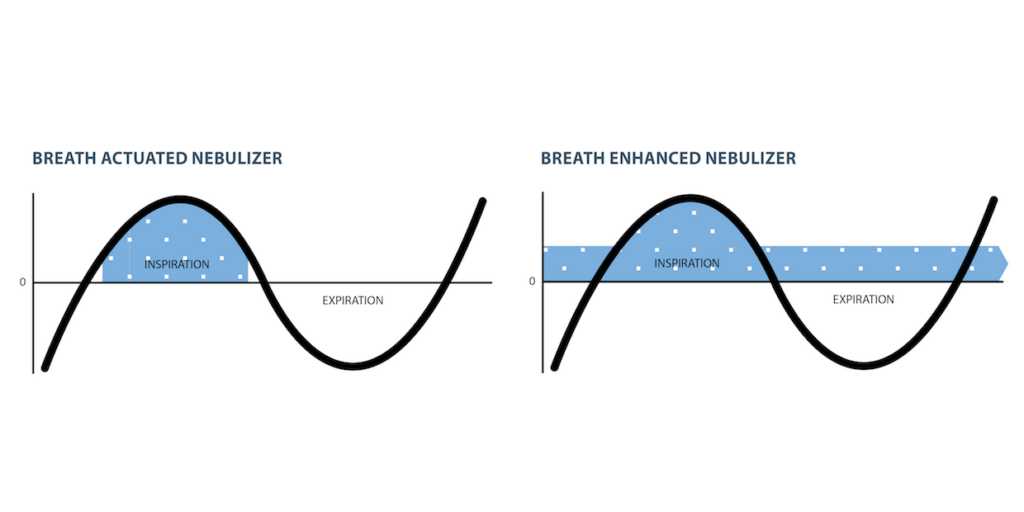
METHODS
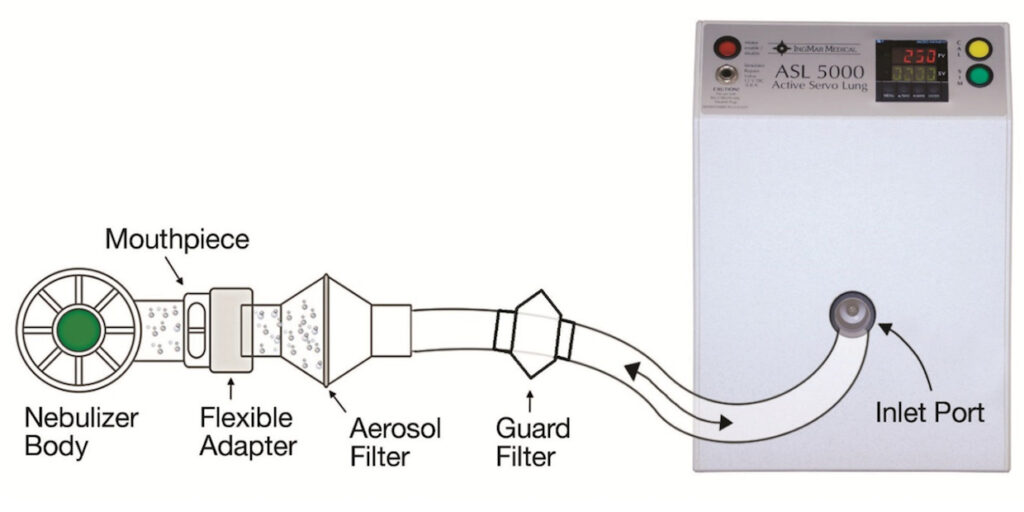
AeroEclipse® XL BAN™ Nebulizer (BA) with Ombra® Table Top Compressor (Trudell Medical International) was evaluated with 300 mg tobramycin (5 mL,Teva-Tobramycin) and an adult tidal breathing waveform (tidal volume = 500 mL; duty cycle = 33%; breaths/minute = 13) delivered by breathing simulator (ASL 5000, IngMar Medical). An electret filter at the nebulizer mouthpiece captured emitted aerosol at minute intervals until onset of sputter. Total mass delivered (TM) was determined. Average delivery rate/min (DRmin) was calculated after assaying for tobramycin by a validated HPLC-based procedure. Parallel measurements of fine droplet fraction <5.4μm diameter (FDF<5.4μm) were made with each nebulizer, sampling the emitted aerosol via a chilled Next Generation Pharmaceutical Impactor at 15 L/min.
Fine droplet mass delivery/min (FDM<5.4μm/min) was determined as the product of DRmin and FDF<5.4μm. Fine droplet mass (FDM<5.4μm) was determined as the product of TM and FDF<5.4μm. Similar measurements were undertaken with PARI LC PLUS† (BE) with DeVilbiss† Pulmo-Aide† compressor and PARI LC PLUS† (BE) with PARI Vios† compressor.

RESULTS
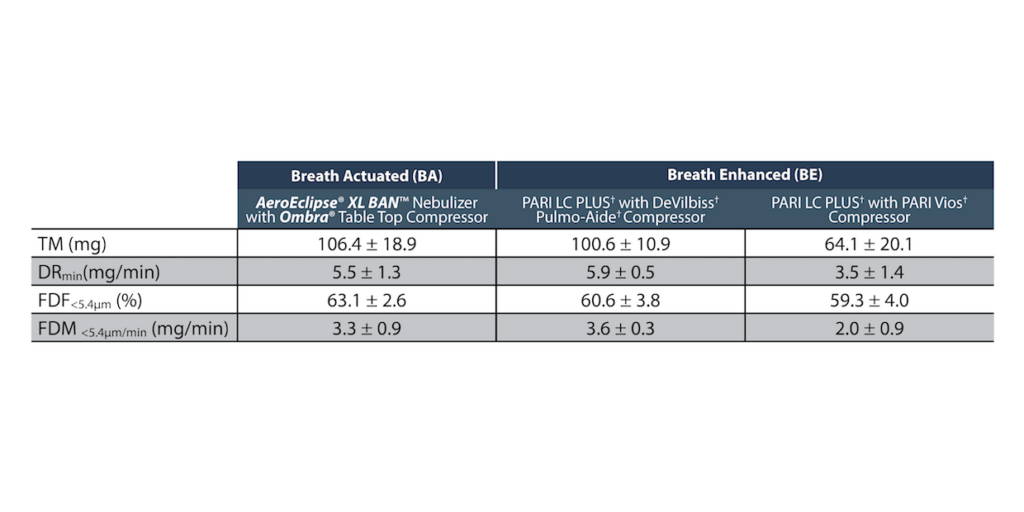
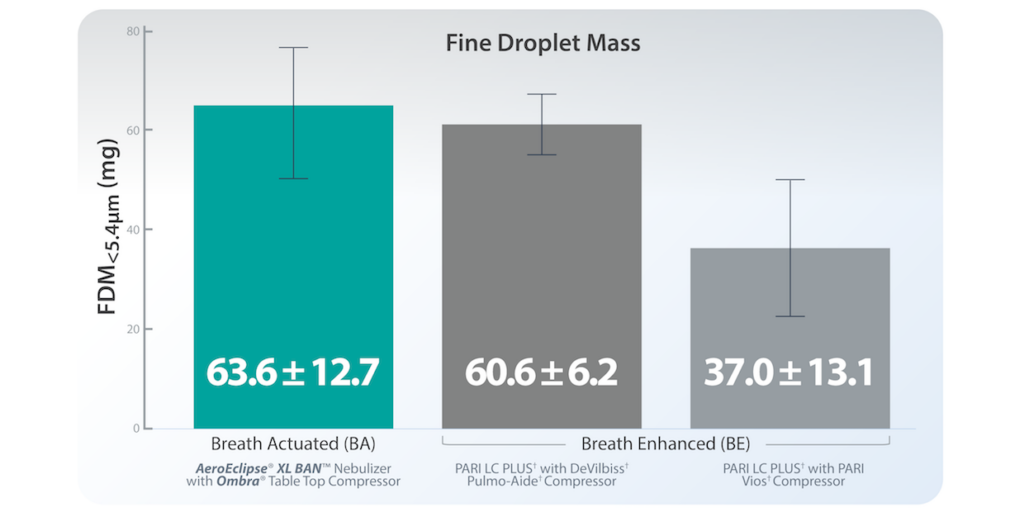
FDM<5.4μm/min data was similar for the BA/compressor system and one of the BE/compressor systems. However, FDM<5.4μm for the BA/compressor system was higher than both BE/compressor systems. (mean ± SD; n= 5 devices/group)
CONCLUSIONS
The breath actuated system performs similarly or better than both breath enhanced systems. The more significant difference in fine droplet mass delivered between the two BE systems may be due to the compressor. On the basis of this study, clinicians could select AeroEclipse® XL BAN™ Nebulizer with Ombra® Table Top Compressor for tobramycin delivery, with the added value of a breath actuated device offering improved dosing consistency1 and low fugitive emissions.2
American Thoracic Society Conference
May 19 – 24, 2023
1 Nagel M, Hoffman N, Suggett J, Wang V. American Journal of Respiratory and Critical Care Medicine 2021;203:A4672. 2 Nagel M, Suggett J, Mitchell JP. Respiratory Drug Delivery 2021;1:287-292. MD-266A-0323
* trademarks and registered trademarks of Trudell Medical International (TMI). † trademarks of their respective companies. ©TMI 2023.
